Tandem azaisobenzofurans formation – Diels-Alder reactions: Applications to the synthesis of biologically active aromatic heterocycles
Prof. Binay Krishna Ghorai
Department of Chemistry
Indian Institute of Engineering Science and Technology, Shibpur
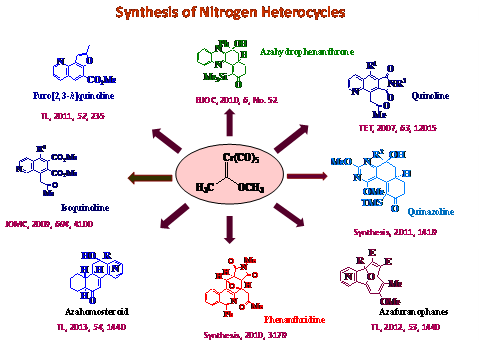
Reactions involving Fischer carbene-chromium complexes and alkynes have emerged as a powerful tool for synthetic organic chemistry, allowing for the direct synthesis of a variety of structural types including aromatic rings and various bicyclic structures. The o-quinoid 10-electron isobenzofuran system has remained the subject of intense theoretical, structural and reactivity studies, but little work has been done with the analogous azaisobenzofuran systems. The primary objectives of the project were as follows: 1.Study of the scope and limit of the tandem furo[3,4-c]isoquinoline intermediate formation by the coupling of appropriate alkynyl carbonyl derivatives with Fischer carbene complex and trapping with Diels-Alder dienophiles: an approach to nitrogen containing heterocyclic analogs of 1-arylisoquinoline lignans. 2.To examine the generation of furo[3,4-d]pyrimidine intermediate by the coupling of appropriate alkynyl carbonyl derivatives with Fischer carbene complex: an approach to nitrogen containing heterolignans. 3.Studies on the generation of furo[3,4-b]quinoxaline intermediate by the coupling of appropriate alkynyl carbonyl derivatives with Fischer carbene complex: an application to the synthesis of phenazine derivatives. 4.Synthesis of heteroatom containing furanophane derivatives through [8+2]- cycloaddition of dienylisobenzofurans and alkynes. 5.Synthesis of heteroatom containing steroid ring systems by coupling of alkynyl carbonyl derivatives with suitably substituted Fischer carbene complex.
